Abstract
Introduction: Severe coronavirus disease 2019 (COVID-19) is associated with thrombotic complications, despite prophylactic and therapeutic anticoagulation. The disease course of critically ill COVID-19 patients has been characterized by severe hypercoagulability and hypofibrinolysis leading to an increased risk of venous thromboembolism. In COVID-19 (low molecular weight) heparins do not provide sufficient protection against venous thromboembolism. Since the start of the COVID-19 pandemic, several immune modulatory interventions have been introduced to improve outcome, including dexamethasone and tocilizumab. Whether dexamethasone and tocilizumab would alter the course of hypercoagulability by modifying inflammation, is unknown. Tocilizumab, that reduces IL-6 binding to its cellular receptors, has been shown to reduce the plasma plasminogen activator inhibitor-1 (PAI-1). On the other hand, dexamethasone can induce PAI-1 release. Data on the net impact of dexamethasone and tocilizumab on fibrinolysis in critically ill COVID-19 patients is lacking. Therefore, we aimed to characterize the fibrinolytic profile of severe COVID-19 patients on different treatment regimens and to unravel underlying mechanisms of the hypofibrinolytic state of these patients.
Methods: A nested case-control study within the Maastricht Intensive Care COVID (MaastrICCht) cohort on mechanically ventilated COVID-19 patients was conducted. Patients receiving tocilizumab and dexamethasone (toci group) were age- and sex-matched with patients receiving only dexamethasone (dexa group) and patients receiving neither tocilizumab nor dexamethasone (control group). Whole blood was collected from these patients at three different time points after intubation (week 1, 2, and 3). The dexa and toci group received dexamethasone treatment before intubation and the toci group received one bolus of tocilizumab before or on the day of intubation. All blood samples were collected thereafter. Whole blood tissue plasminogen activator rotational thromboelastometry (tPA-ROTEM) was performed and subsequently inflammatory markers (c-reactive protein (CRP), ferritin, IL-6, fibrinogen), fibrinolysis parameters (tPA, PAI-1, thrombin activatable fibrinolysis inhibitor (TAFI), plasmin-alpha-2-antiplasmin complex (PAP)) and coagulation biomarkers (D-dimer, prothrombin fragment 1.2 (F1.2)) were measured in plasma samples. All markers were analyzed with linear mixed-effects regression models to estimate the differences between treatment groups over time.
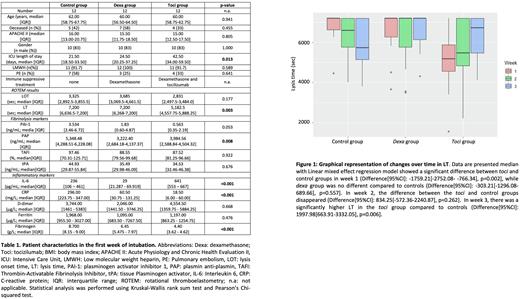
Results: Matching showed similar groups (Table 1.) for the baseline characteristics. At week 1 the dexagroup's IL-6 level was significantly lower, whereas the toci group's IL-6 level was significantly higher compared to the control group (difference[95%CI]: -198.37 [-350.86- -45.88], p=0.017 and 269.59 [113.91-425.27], p=0.002, respectively). CRP levels were significantly lower at week 1 in both dexa and toci groups compared to the control group (median [IQR] 60.50 [30.75 - 131.25] and 18.50 [6.00 - 60.00] vs. 296.00 [223.75 - 347.00]) suggesting effective immune suppression. Divergent fibrinolysis was seen at week 1 by tPA-ROTEM showing lower lysis time (LT) in the toci group compared to the control group (difference[95%CI]: -1,759.21 [-2752.05 - -766.34; p=0.002), whereas the dexa group did not differ. Interestingly, LT decreased over time in the control group, while an increase was seen in the toci group (Figure 1.). PAI-1 levels were lower in week 1 in the toci group compared to the control group (difference [95%CI] -7.32 [-14.95 - 0.30], p=0.071) and the tocigroup showed an overall increase in PAI-1 levels, while the control and dexa group showed a decrease over time. High TAFI and tPA levels were observed in all groups at week 1 and over time regardless of the treatment regimens. Coagulation biomarkers D-dimer and prothrombin fragment 1.2 were elevated but did not differ or change over time between groups.
Conclusions: The differences and changes over time revealed by tPA-ROTEM and the fibrinolysis markers between the three different treatment regimens show the direct interaction between inflammation and fibrinolysis in these patients. The lower LT and PAI-1 levels upon tocilizumab treatment could be indicative for better fibrinolytic activity in these patients.
This study was supported by the Dutch Covid and Thrombosis Coalition (DCTC).
Disclosures
ten Cate:Alveron Pharma: Consultancy; Bayer AG: Research Funding; Pfizer: Research Funding; Astra Zeneca: Research Funding; Leo Pharma: Research Funding; Alexion: Research Funding; Galapagos: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal